Beekeeper-funded Research
A Test of Thermal Treatment for Varroa
Part 2
First published in ABJ April 2021
Randy Oliver
ScientificBeekeeping.com
Last month I covered the theory of using thermal treatment (hyperthermia) to control varroa, and some of the designs of devices on the market. I’d like to now answer some questions regarding thermal treatment. I also asked users for reports on their observations, and tested thermal treatment myself on four hives.
Effects of Heating upon the Workers
Allow me to quote from the manufacturers of the Thermosolar Hive [[1]].
Q: Can the thermotherapy harm the colony?
A: No. The upper limit for thermotherapy is 47°C (116.6°F). The temperature might rise maximally by 1 – 2°C (1.8 – 3.6°F) for a few minutes after the roof is put back…The adult bees have the chance to move to the bottom box, bottom board or to leave the hive. Relative humidity of the air decreases during the heating; the bees tolerate the dry air easily. The house bees withstand the higher temperatures easily as well, they remain on the heated brood during the whole therapy… The brood remains intact, healthy bees do hatch even at 50°C (122°F). It is not recommended to exceed the healing temperatures (40 – 47°C, 104 – 116.6°F), nor the duration of the treatment (2,5 hours).
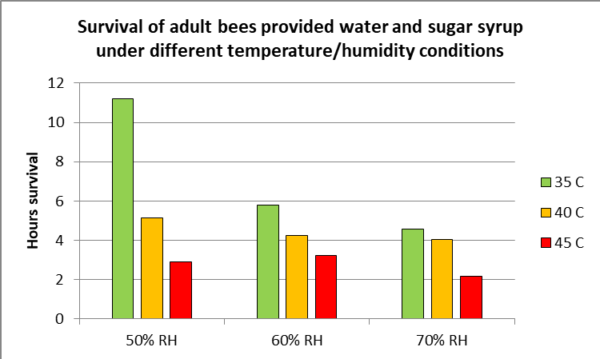
Now compare the above answer to the hard data from a recent study (Figure 1).
Fig. 1. Note how dramatically the survivorship of adult bees drops as the temperature rises from 35 C to 45 C (95F – 113 F). These results indicate the effect of humidity of adult bee survival at elevated temperatures. This may be how the bees survive in hyperthermia devices that don’t add humidity — by evaporating water from their mouthparts. Data from Li (2019)[[2]].
The above graph is for exposures of hours. With any thermal device, the heat must be applied gently and evenly, since Kovak [[3]] found that even 5 minutes exposure to 51.7 C (125 F) caused immediate or delayed death of 50% of Italian bees, and that Carniolan bees were even more sensitive.
Practical application: Some races of honey bees are more heat tolerant than others. If you keep “Carnies,” be aware that they don’t handle heat as well as do Italians. And make sure that your thermal device doesn’t create any “hot spots” in the hive.
Neither I, nor other beekeepers I’ve spoken with, notice many dead adult bees after thermal treatment. But that doesn’t mean that there’s no adult mortality, since as a general rule, adult workers that are stressed fly out of the hive to die elsewhere. Free [[4]] found that exposure of caged workers to a temperature of only 40°C (104°F) for 1 hour resulted in some of them dying within the next 24 hours. In any case, the benefit from reducing the mite infestation appears to outweigh the adverse effects upon the colony, and the beekeepers I’ve spoken with generally say that their colonies perform well after thermal treatment.
Effect upon the Brood
The honey bee colony carefully regulates the temperature its brood within a very narrow range. It is reasonable to suspect that heating the brood to a higher temperature my cause “adverse effects” upon the larvae or pupae, either immediately, or later in their lives. There have been a number of studies on this subject, well reviewed by Kablau [[5]], suggesting that we may have more to learn.
As I mentioned in the first part of this series, Kablau found that to effectively kill the mites in the brood, you needed to apply a temperature that also kills drone brood. It’s up to the beekeeper to weigh the tradeoff.
In the hives that I’ve thermal treated (at 41 C), I did notice a few dead older larvae, presumably killed by the heat, but not enough to be concerned about — unless there is a delayed effect upon them once they emerge as adults. I didn’t check for survivorship of eggs or very young larvae, which may suffer not only from heat, but from desiccation.
Effect upon the Drones and Queens
Another concern is the effect upon the queen, since it’s been found that even brief exposure to 40°C (104°F) can cause substantial degradation of sperm viability [[6], [7]]. And it appears that drones are more sensitive to heat than are workers [[8]].
Practical application: We’re learning more and more about how temperature extremes can affect sperm viability, whether in the drone’s testes, or the spermatheca of the recipient queen. I suspect that queen producers may wish to steer clear of thermal treatment.
Beekeeper Doug Severt from Montana, who has used thermal treatment on several hives over the course of three years, reports that he’s noticed some queens failing after thermal treatment, as did I in my tests. Other users tell me that they don’t notice this.
Practical application: Without performing a field trial with marked queens in test and control colonies, it can’t be determined whether thermal treatment actually causes queen failure. And even if it does, as with queen “issues” due to formic acid treatment, colonies may simply rear replacement queens.
Effect upon Small Hive Beetle
A number of beekeepers report that thermal treatment kills Small Hive Beetles (SHB). SHB prefers cooler parts of the hive, and has reduced longevity at broodnest temperature [[9]]. I haven’t been able to find any studies testing the effect of short-term thermal treatment upon SHB.
Does Thermal Treatment Sterilize the Mites?
One manufacturer claims that “Should any of Varroa mites survive, they are so damaged that they can no longer reproduce. “ If this is indeed true, then we shouldn’t see any rebound in the mite population after thermal treatment. We’ll take a look at field data further down to see if this is true.
How about the “Phoretic” Mites on the Adult Bees?
Several beekeepers report that high numbers of mites fall to the bottom of the hive during thermal treatment, but as I pointed out last month, they may still be alive. This would be one advantage to a bottom-plate thermal device, since any fallen mites would perish on the hot plate. But how about the mites on the bees that beard on the front of the hive when using thermal devices that allow them to do so? Lynn Williams used carpenter’s chalk dust to mark the bearded bees, and observed bees moving not only out of the hive, but also back in. Further down I’ll show the change in the mite infestation rate of the adult bees 24 hours after thermal treatment.
Reports from U.S. Beekeepers
I put out the word that I wished to hear from beekeepers who not only have tried thermal treatment, but had also collected some data on its efficacy. Adding the condition that the person had made the effort to collect data helps to sort those with scientific interest from “true believers,” and I enjoyed objective discussions with the beekeepers below. They had all used the popular Mighty Mite device (which has a Facebook page [[10]]). The Mighty Mite is a bottom-inserted, thermostatically-controlled, open-entrance unit, so the data below only reflect the results for this specific device, which holds a temperature of approximately 41 C (106 F) for 2.5 hours.
Mike Immer
Mike [[11]] is a beekeeper from Missouri, who has customers who wanted nucs that had not received any chemical treatments. So he tested thermal treatment on 30 hives in an isolated experimental yard, and graphed his results (Figure 2).
Fig. 2. Mike Immer’s alcohol wash counts prior to, and following, thermal treatment of 30 hives with the Mighty Mite during the summer. These results were impressive! Mike also reported a substantial reduction in SHB counts.
Mike also related to me that he tested the device again the next year on four hives in a different yard, and that the results were not quite as impressive. You can read more about Mike’s experience with thermal treatment here [[12]].
Stickyboard Counts
Mike took alcohol wash counts from samples of adult bees. Such counts accurately reflect the infestation rate of the workers, which shows strong biological relevance with regard to the degree of viral infection of the bees [[13]]. That said, the next two data sets, instead of being mite wash counts, are of 24-hour stickyboard counts of natural mite fall.
Although stickyboard counts are used by many beekeepers for mite monitoring, they are more difficult to interpret, since they exhibit far more day-to-day variation than do mite washes [[14]], and must be interpreted relative to colony size and amount of brood present. Unlike mite wash counts, for which there is plenty of data that shows that once the infestation rate exceeds 5% (~15 mites per half cup of bees) that colony performance and survivability begins to seriously suffer, extension agents can only give ballpark figures as to seasonal “economic damage thresholds” based upon stickyboard counts. My Google search found a treatment threshold of 50 mites per day late in the season to be a common recommendation. That figure astounds me, since at the normal rate of natural attrition of varroa of about 1% of the varroa population per day [[15]], a drop count of 50 would indicate an in-hive population of 5,000 mites — 5x higher than the 1000-mite maximum recommended in Europe. A more realistic treatment threshold is suggested by Dr. Yves Le Conte [[16]]:
Today, German beekeepers are required to start treatment if the natural mite drop exceeds 10 mites per day, a level that indicates the colony is close to collapse.
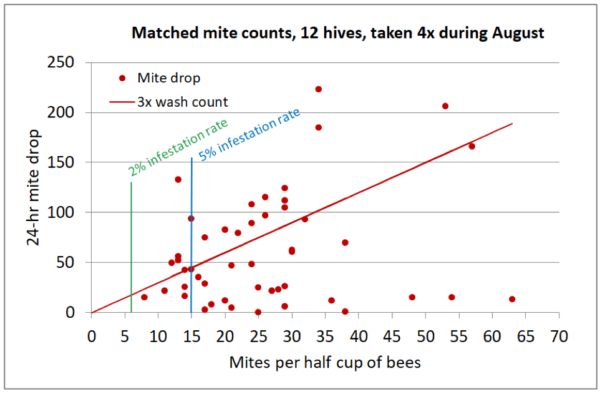
There is unfortunately no straightforward conversion between the two monitoring methods, and natural mite fall may greatly underestimate the actual infestation rate of the colony (Figure 3).
Fig. 3. A plot of data that I collected a few years ago, from 12 double-deep hives, averaging 13 frames of bees, during August. Although there is only a very rough correlation between infestation rates and mite drops, I eyeballed a regression line of 3x the alcohol wash count, ignoring the few outlier hives that exhibited unexpectedly low mite drops. Note that stickyboard counts can greatly underestimate the infestation rate of the adult bees in the hive.
In the above graph I inserted two mite wash infestation rates —the 2% rate is the treatment threshold that we use in our operation; above 5%, colony performance and winter survival start to drop off. Note that my graph above exactly matches the German recommendation of 10 mites dropping per day for a treatment threshold at the 2% infestation rate, and that 50 mites dropping per day would indicate an infestation rate of above 5%.
Practical application: I’m surprised to see Extension webpages suggesting that a late-season 24-hr stickyboard count of 50 mites would be acceptable. I’d treat by the time 10 mites start dropping per day.
With the above information in mind, let’s see what we can learn from some stickyboard counts.
Carl Chesick
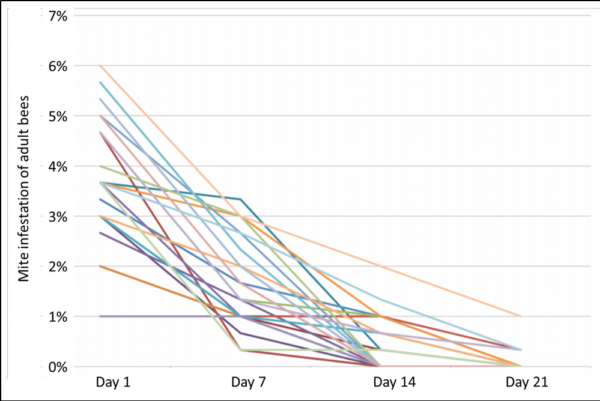
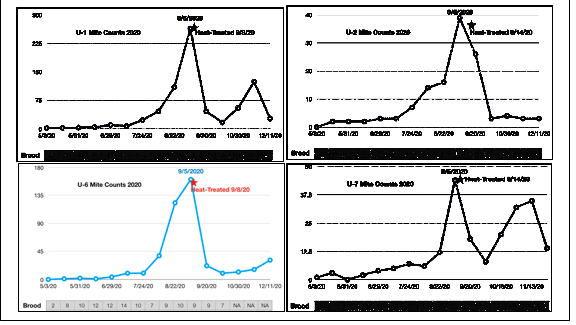
Carl heads the citizen science Center for Honey Bee Research in South Carolina. As opposed to Mike, who took mite wash counts of the adult bees, Carl’s group recorded stickyboard counts of mite fall. Below are representative graphs from four of the ten that he sent me, all having been given a Mighty Mite thermal treatment on September 5 (Figure 4).
Fig. 4. Stickyboard counts (24-hr) from representative hives given thermal treatment (note that the y-axes are of different scales). I picked hives that contained a substantial amount of brood at time of treatment, in order to see whether there was much mite recovery post treatment.
Practical application: Thermal treatment in early September definitely caused major reductions in natural mite fall, and was able to at least temporarily reduce the daily count to 10 or less in six of the ten treated hives. But also in six of the ten hives, the mite drop counts were back at dangerous levels by November, indicating that a thermal treatment in early September might not prevent virus issues in the population of “winter bees.”
Kay
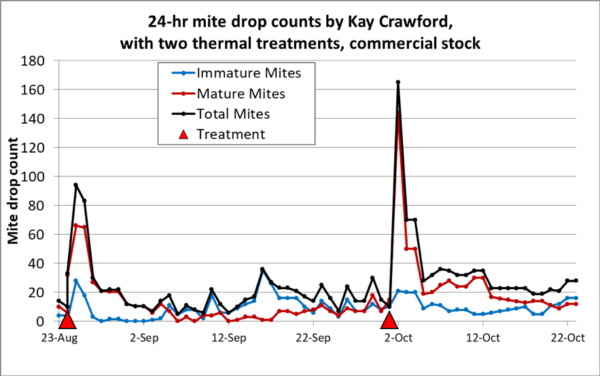
Beekeeper Kay Crawford in Washington kept track of the effect of thermal treatments by a Mighty Mite device in two Slovenian AŽ hives (hives kept in a shed, with the frames pulling out horizontally). Her data were also of natural drop on a stickyboard, but Kay went a bit further, and counted immature mites separately from dark mature mites, and took counts more frequently (Figure 5).
Fig. 5. Mite drop counts for a colony headed by a commercial queen. Each treatment clearly caused a spike in mite fall, especially of mature mites (note the shift in the red plot compared to the blue plot). But this data set indicates that thermal treatment didn’t cause much of a change in mite drop counts from the baseline values prior to treatment. Nor did it reduce the mite drop to a level that would indicate good mite control.
Having done a fair number of stickyboard counts myself, I was surprised by the number of immature mites that Kay counted. So I checked with Dr. Lilia De Guzman, who during her tenure at USDA, has perhaps counted (and dissected) as many varroa mites as any human being. I’ve always been impressed by Dr. De Guzman’s meticulous research, and reviewed a study in which she had recorded the proportions of older, younger, and nymphal mites. From colonies engaged in broodrearing, she recorded that 42% of fallen mites fell into the young or nymph class. So Kay’s counts are not surprising. However, of considerable interest, we can use her counts to see how thermal treatment affected the “phoretic” mites compared to those emerging immature from the brood.
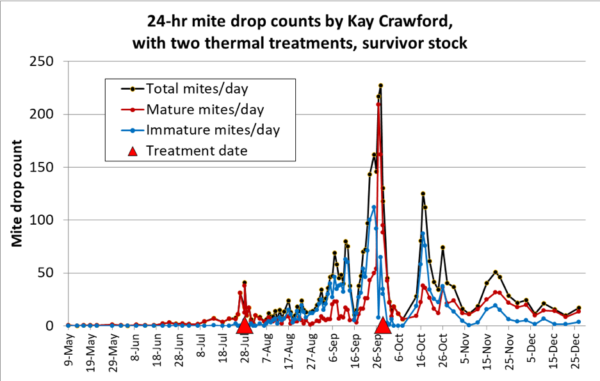
Kay also treated a hive headed by a “survivor stock” queen (Figure 6).
Fig. 6. Daily mite drop counts (some averaged from multi-day counts) of a “survivor stock” colony over a season, punctuated by two thermal treatments. Again, note that hyperthermia appeared to cause elevated mortality of mature “phoretic” mites for a period of time post treatment. By mid-October, the mites appear to have resumed another round of vigorous reproduction, tapering off in December.
Practical application: So just how efficacious were the thermal treatments, as far as reducing the mite population? I used my varroa model [[17]] to see if I could come up with a rough estimate, based upon Kay’s mite drop data, to estimate the efficacy of the treatments in the graphs above. It suggests that each treatment killed around 40-50% of the mites in the hive. The model also indicates that 40-50% would have been the proportion of “phoretic” mites in the colony at that time, supporting the explanation that the treatment mainly hit the mites on the adult bees. The relatively rapid rebound of the mite populations after treatment is further evidence suggesting that a healthy population of mites remained in the colony post treatment.
My own Preliminary Investigation of a Thermal Treatment
At the request of beekeeper Bill Hurd, who sent me a thermal unit to test, I did some quick and dirty preliminary investigations of thermal treatment.
Disclaimer: I did not run an actual scientific experiment to test efficacy of thermal treatment, but rather performed only a preliminary investigation on:
- Using the method in the field,
- Confirmation of temperature penetration of the combs,
- To determine the impact of treatment upon the course of mite infestation rate of the adult bees following treatment,
- To determine the short-term mortality of mites in the brood following treatment.
For the investigation, I used a thermostatically-controlled and timed heating plate installed on the bottom board, with temperature monitored by a thermistor set on a central top bar of the lower brood chamber. After some practice rounds, I treated four moderate-strength hives, all double-deep with ample brood, exhibiting relatively high mite counts. I performed the treatments July and August, during daytime, with ambient temperatures ranging from 85 to 105 F (Figures 7-9).
Fig. 7. I taped any cracks between the brood chambers, and weighted a piece of insulation board tight against the propolis at the top of the hives. Many of the adult bees chose to move out of the hive as it warmed up.
Fig. 8. I used a separate thermistor from that of the heating unit to monitor the temperature between the combs of the upper box. Although I used the same device and setup for each hive, in one run the temperature of the upper box only reached 101 F, perhaps due to the ambient temperature being only 86 F.
Fig. 9. I also inserted a thermistor beneath sealed brood, against the midrib of a comb in the middle of the upper box, in order confirm that the heat penetrated to the bottoms of the cells (it did).
Dissection of the Brood after Treatment
In researching thermal treatment, at the Varroa Controller website I’d found an interesting observation. Dr. Stefan Berg [[18]] writes (roughly translated from German):
If you heat the brood combs to 41 C for two hours, the young daughter mites and the males are killed, but the mother mites survive this quite well. After the treatment we plucked brood, only ten percent of the mother mites were damaged. At three hours and 42 C almost all of the mites were dead.
Since in my test, I’d heated to 41 C for 2½ hours, I was curious as to how what percentage of the mature female mites under the cappings survived the sauna experience (Figures 10-11, Table 1).
Fig. 10. It was easy to determine whether the mature (dark colored) female mites had been killed or not by their unmistakable rapid movement, or lack thereof. In some of the drone cells that I dissected, there were as many as five mature mites. I couldn’t find mites in worker cells in one of the hives. I dissected both solid patches of cells, as well as cells next to empty cells (which would allow better heat penetration), and didn’t notice any difference in mite mortality.
Fig. 11. I pulled brood frames from the upper box, 24 or 48 hours after thermal treatment, and dissected out 5th-instar larvae and pupae of various ages. This photo shows drone brood that I pulled from the patch of cells above the plate. You can’t tell from the photo, but to my surprise, most all the mites were actively walking about.
A summary of my dissection data is shown in Table 1.
| Table 1. I recorded the counts of live or dead mites from combs from three of the test hives. In only one row of drone cells was the number of dead mites greater than the number of live ones. Of the 153 mites that I pulled out, only 29% of them were dead. | |||||
| MITE SURVIVORSHIP POST TREATMENT | |||||
| All inspected cells from a central frame in the upper brood chamber. | |||||
| Hive (temperature held) Time of dissection | Drone | Worker | |||
| Live | Dead | Live | Dead | ||
| T1 (106-107 F) 24 hr | 2 | 16* | 22 | 2 | |
| T2 (107 F) 24 hr | 34 | 3 | 0 | 0 | |
| T2 (107 F) 48 hr | 12 | 4 | n/a | n/a | |
| T3 (101 F) 24 hr | 40 | 8** | 9 | 1 | |
| * These drone cells were along the bottom bar of the upper brood chamber.
**In this run, the upper box temperature did not exceed 101 F. |
|||||
Practical application: My observations support those of Dr. Berg. A 2.5-hour treatment at 106 F is not adequate to obtain a high rate of kill of the mature mites in the brood, even at 48 hours after treatment.
This leaves the question as to the reproductive capacity of the surviving mites post treatment. If they are all sterilized, we wouldn’t expect the mite population to rebound. The mite fall data reported above suggests that the surviving mites are indeed able to reproduce. So let’s take a look at my alcohol wash counts.
The Effect of Treatment upon the Mite Infestation Rate of Adult Bees
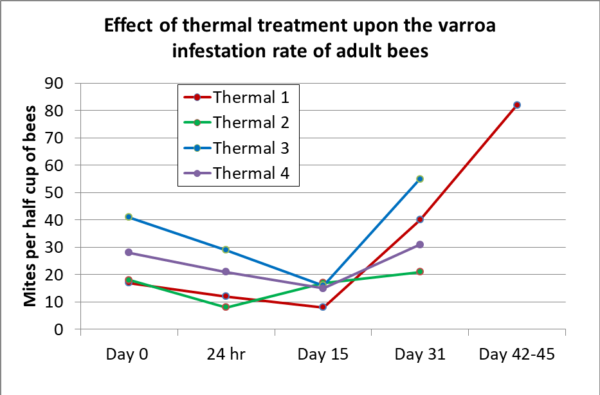
I performed mite wash counts for each hive before treatment, at 24 hours post treatment, and then at Days 15 and 31. In addition, for the first treated hive, I took an additional count at Day 42. I took the bee samples from the same marked frame (immediately adjacent to brood) each time in each hive in order ensure consistency [[19]] (Figure 13).
Fig. 13. In no case did my results match those of Mike Immer. Surprisingly, the average reduction in infestation rate of the adult bees at 24 hours post treatment was only 35%, and by Day 15 only 41%. And then the mite counts shot right back up, indicating that the reproductive ability of the surviving mites was not hampered to any great extent. Note that the upper-box of Thermal 3 held at only 101 F (38 C), but surprisingly showed the greatest reduction in mite count. Also of interest is that its mites didn’t recover any faster than in Thermal 1, which held at 107 F (41.7 C).
My modeling these results suggests that the overall kill rate of the total mite population in the hive ranged from around 40% to 55%.
Practical application: I applied a 106 F for 2.5 hours thermal treatment regimen to three of these hives, under ideal conditions (hot weather), confirming both the temperature held, and duration of, the treatment, as well as the penetration of the heat to the midrib of the brood combs in the upper box. My results are in agreement with the “thermal death time” curves in Fig. 4 of my previous article — that this regimen would be very well-tolerated by the bees and brood, but unfortunately fairly well-tolerated by adult mites also. So yes, it indeed killed a proportion of the mites, but only enough to set their buildup back by a month to a month and a half.
Discussion
The honey bee colony maintains its broodnest within a consistent and very narrow range of temperature and humidity. For successful thermal treatment — meaning that you’d kill most of the mites, with minimal damage to the colony as a whole — we’re dealing with a very narrow range of temperature and time. Too low a temperature, or too short a time, and mite control will be inadequate. But only a few degrees higher, or an hour longer, and the brood can be damaged, and the queen and drones could potentially be largely sterilized. And even then, the adult bees that move outside to escape the heat take their hitchhiking mites with them, and may then carry them back in when it’s safe. Since half the mites are typically on the adult bees, this is an entrenched limitation to thermal treatment, unless coupled with a different treatment to kill those mites.
Practical application: Hyperthermia of hives has the potential to greatly reduce mite levels, but the trick will be for the device and treatment regimen to hone in on the “sweet spot” of time and temperature that will kill most of the mites, with the least damage to the bees.
FWIW, hyperthermia is far more time-consuming and labor-intensive than cracking open a hive and tossing in a treatment. All devices require a power source — the grid, generator, battery, or solar. Thermal treatment could be used by the small-scale beekeeper who is adamantly against using any “substance” for mite control, but it’s hard for me to imagine it being cost effective for beekeepers with many hives.
Acknowledgements
Thanks to Bill Hurd, Lynn Williams, Mike Immer, Carl Chesick, Amy Muscante, Kay Crawford, Darwyn Flynn, Doug Severt, Samuel Ramsey, and Lilia De Guzman for their research efforts and helpful correspondence. And to Peter Borst for his research assistance. A tip of the hat to Patricia Loreto Aldea Sánchez for her instructive thesis.
I’d like to honor the passing of my friend Aaron Morris, who moderated the Informed Discussion Group of Beekeeping Issues and Bee Biology (Bee-L) until his recent passing. The beekeeping community is full of selfless individuals who give all they’ve got for the benefit of the rest of us.
Citations
[1] https://www.thermosolarhive.com/faq/
[2] Li X, et al. (2019) Tolerance and response of two honeybee species Apis cerana and Apis mellifera to high temperature and relative humidity. PLoS ONE 14(6): e0217921.
[3] Kovac H, et al (2014) Metabolism and upper thermal limits of Apis mellifera carnica and A.m.ligustica. Apidologie. 45(6):664–677.
[4] Free, JB & Y Spencer-Booth (1962) The upper lethal temperature of honeybees. Entomologia Experimentalis et Applicata 5(4): 249-254.
[5] Kablau, A, et al (2020) Hyperthermia treatment can kill immature and adult Varroa destructor mites without reducing drone fertility. Apidologie 51:307–315.
[6] Stürup, M, et al (2013) When every sperm counts: factors affecting male fertility in the honeybee Apis mellifera. Behavioral Ecology 24(5): 1192–1198.
[7] Pettis, J, et al (2016) Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS ONE 11, e0147220.
[8] McAfee, A, et al (2020) Vulnerability of honey bee queens to heat-induced loss of fertility. Nat Sustain 3, 367–376.
[9] Meikle, W. G., & Patt, J. M. (2011). The effects of temperature, diet, and other factors on development, survivorship, and oviposition of Aethina tumida (Coleoptera: Nitidulidae). Journal of Economic Entomology, 104(3), 753–763.
[10] https://www.facebook.com/groups/275791919813444/
[11] Mike Immer www.BeeResQ.com
[12] https://www.beeresq.com/post/thermal-control-of-varroa-and-shb-blog-version-pictures-and-graphs-removed
[13] Francis, RM, et al (2013) Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 8(3): e57540
[14] http://scientificbeekeeping.com/mite-management-update-2013/
[15] Branco, MR, et al (2006) A comparative evaluation of sampling methods for Varroa destructor (Acari: Varroidae) population estimation. Apidologie 37: 452–461.
[16] Le Conte, Y, et al (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41(3): 353 – 363.
[17] http://scientificbeekeeping.com/randys-varroa-model/
[18] https://www.varroa-controller.com/wp-content/uploads/2020/06/dbj-2017-1-Seite-16-17.pdf
[19] http://scientificbeekeeping.com/re-evaluating-varroa-monitoring-part-3/